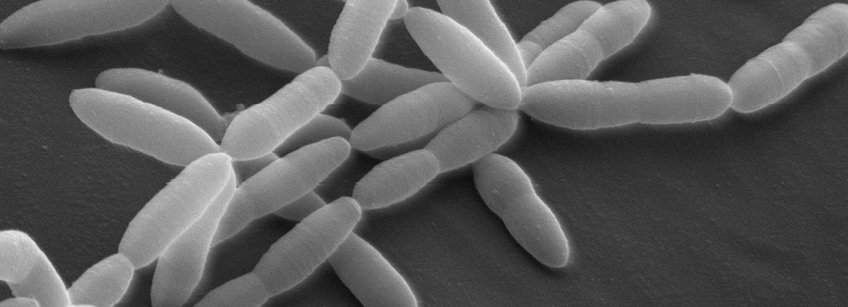
Microbiome Science - Ruth E. Ley
The Department of Microbiome Science is broadly interested in the ecology and evolution of the human gut microbiota. We perform population-level research to probe links between human genotype and the gut microbiota, and we focus mechanistically on ways in which specific gut microbes have adapted to the human body.
In the Department of Microbiome Science, we ask fundamental questions about the evolutionary origins of the human gut microbiome and how it influences host physiology and evolution. We have the following main areas of research: (1) evolution of the human gut microbiome and interplay with host genetics, (2) lipids in host-microbiome symbiosis, (3) microbiota-innate immune interactions, (4) development of genetic systems for currently intractable microbes. Please see the representative papers from the lab in these areas below, and visit our Department’s website for more information LEY LAB.
Press releases & research news
Ruth Ley, Detlef Weigel and Postdoc William “Tony” Walters are once again among Clarivate’s ranking of the top 1% by citations in their respective fields on the Web of Science™. For the past ten years, Ruth Ley, Director of the Department of Microbiome Science, and Detlef Weigel, Director of the Department of Molecular Biology, have been recognised as recipients of the Highly Cited Researcher Award. For William Anthony Walters “Tony”, a postdoc in the Department of Microbiome Science, this will be his fourth consecutive year to receive this recognition.
more
The SynTracker tool expands traditional microbial analysis by considering genomic structural variation to complement existing SNP-based methods. This innovation reveals more precision and depths of microbial strain diversity and evolution. SynTracker empowers scientists with species-specific analysis capabilities, allowing them to focus on genome synteny of microbial populations.
more
Our bodies constantly encounter bacteria, especially in our gut and lungs. To fight harmful bacteria, we have immune system soldiers called Pattern Recognition Receptors (PRR) that recognise flagellins produced on bacteria as enemies. Flagellins are tail-like proteins that facilitate tissue invasion and are a common feature of harmful bacteria. One PRR is TLR5, which detects flagellins and triggers inflammation to fight them.
more
Max Planck Institute Director receives funding for Silent Flagellin in Chronic Inflammatory and Auto-immune Disease (SilentFlame). This funding will enable Ley and her team to investigate how flagellins produced by beneficial gut bacteria interact with the human immune system.
more